Development of novel PET radiotracers for immunoPET imaging of heart and brain
The recent advances in antibody engineering has made it possible to construct antibodies with ultimate target specificity and tailored pharmacokinetics. Besides their extensive use for targeted therapies, recombinant antibodies are critical tools in diagnostic immuno PET imaging in oncology, as well as are driving exciting developments in the emerging field of PET imaging for CNS and cardiovascular diseases. This research project focuses on immuno PET imaging of novel molecular targets related to the health of heart and brain and exploring the possibilities of novel pretargeted PET radiopharmaceuticals.
Key words:
ImmunoPET, PET imaging, PET radiotracers, Pharmacokinetics, Heart health, Brain health
Overview of the Research Project and Collaborative Environment:
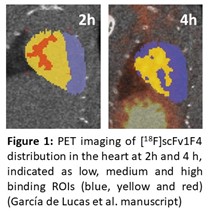
The project builds on the combined expertise of two laboratories utilizing the most recent technologies in antibody engineering and PET radiochemistry, respectively, to develop pharmacokinetically tailored recombinant antibody fragments for PET imaging of target proteins in the heart and brain. Our laboratories possesses a comprehensive array of tools for the discovery and engineering of novel recombinant antibodies. These include high-performing human antibody phage libraries, bacterial and mammalian expression systems, and efficient methods for purification and characterization, allowing us to engineer antibodies in various formats for diverse applications. Moreover, we have developed radiosynthetic chemistry for labelling of antibodies and other biomacromolecules in mild reaction conditions with positron emitters, such as 18F, 68Ga and 89Zr. The developed methods have been successfully utilized for evaluation of new PET tracer candidates for pretargeted and targeted PET imaging. As an example, by joined research efforts of our groups, we have successfully created a 1st generation of novel antibody fragment based tracers specific to the GABA-A a1 receptor subtype. [18F]scFv1F4 and [89Zr]di-scFv1F4 were developed for imaging GABA-A a1 receptor subtype in peripheral organs and in the brain. From these the bispecific [89Zr]di-scFv1F4 was able to cross the blood-brain barrier. The single fragment, [18F]scFv1F4, exhibited favorable pharmacokinetics and specific signal in the heart with areas with highest expression of the α1 subunit of GABA-A receptors, with highest activity 2h after the injection (Figure 1).
The candidate should have:
- PhD in a suitable field (chemistry, radiochemistry, medicinal chemistry, biochemistry or biotechnology)
- Previous research experience in new radiotracer development. In addition, expertise in recombinant protein expression and/or purification is deemed advantageous.
Selected publications:
- Ángel García de Lucas, Urpo Lamminmäki, and Francisco R. López-Picón. 2023. "ImmunoPET Directed to the Brain: A New Tool for Preclinical and Clinical Neuroscience" Biomolecules, 2023, 13, no. 1: 164.
- Tatsiana Auchynnikavaa, Antti Äärelä, Heidi Liljenbäck, Juulia Järvinen, Putri Andriana, Luciana Kovacs, Jarkko Rautio, Johan Rajander, Pasi Virta, Anne Roivainen, Xiang-Guo Li, Anu J. Airaksinen. Tetrazine glycoconjugate for pretargeted PET imaging of trans-cyclooctene functionalized molecular spherical nucleic acids. ACS Omega, 2023, https://doi.org/10.1021/acsomega.3c04041
- Dave Lumen, Danielle Vugts, Marion Chomet, Surachet Imlimthan, Mirkka Sarparanta, Ricardo Vos, Maxime Schreurs, Mariska Verlaan, Pauline Lang, Wissam Beaino, Albert D. Windhorst, Anu J. Airaksinen. Pretargeted PET imaging with a TCO-conjugated anti-CD44v6 chimeric mAb U36 and [89Zr]Zr-DFO-PEG5-Tz. Bioconjugate Chemistry, 2022, 33, 956-968
Anu Airaksinen
Professor
Turku PET Centre/Department of Chemistry
Urpo Lamminmäki
Professor
Department of Life Technologies
Computational analysis of multimodal spatial measurements of cardiovascular disease
Advances in measurement technologies have enabled collecting high-resolution data from multiple modalities, including genomics and histopathology – the vast amount of multimodal data paves the way towards improved understanding of disease mechanisms as well are treatment. Together with our collaborators, we will develop computational methods towards unveiling mechanistic basis of Atherosclerotic Cardiovascular Disease (ASCVD) and cardiometabolic diseases using multimodal and spatial data from cardiovascular tissues. We will especially focus on developing computational methods and tools for integration and joint analysis of histology and cardiovascular genomics. Deep learning based methods are developed for accurate single-cell level analysis, and feature representations are combined with single-cell multiomics and spatial transcriptomics data to gain novel insight into the cardiovascular disease mechanisms.
Our research group has extensive experience in biomedical image analysis, especially in digital pathology. We build computational systems using modern machine learning and artificial intelligence and utilize high-performance computing. We are a multinational team of enthusiastic and skilled computer scientists with diverse cultural background. Our group operates in close collaboration with biologists, pathologists, medical doctors and bioinformaticians, and our collaboration network consists of highly ranked researchers and laboratories from Finland and internationally. We are part of the immunology related InFlames flagship.
We are looking for highly motivated PhD with degree from computer science or related field, and experience from computational biology and image analysis. Successful candidate has skills in Python programming and ability to handle large datasets as well as run large-scale computing. The role of the post-doctoral researcher is to lead computational method development and to operate in close collaboration in a highly multidisciplinary and international team with outstanding collaborators who are leading experts in cardiovascular genomics.
Key words:
AI, histology, genomics, spatial analysis, multimodal data, cardiovascular disease, atherosclerosis
Selected publications:
- Bulten, W., Kartasalo, K., Chen, P. H. C., Ström, P., Pinckaers, H., Nagpal, K., … Ruusuvuori, P., Litjens, G., & Eklund, M. (2022). Artificial intelligence for diagnosis and Gleason grading of prostate cancer: the PANDA challenge. Nature medicine, 28(1), 154-163.
- Valkonen, M., Isola, J., Ylinen, O., Muhonen, V., Saxlin, A., Tolonen, T., ... & Ruusuvuori, P. (2019). Cytokeratin-supervised deep learning for automatic recognition of epithelial cells in breast cancers stained for ER, PR, and Ki-67. IEEE transactions on medical imaging, 39(2), 534-542.
- Wang, Y., Kartasalo, K., Weitz, P., Acs, B., Valkonen, M., Larsson, C., Ruusuvuori, P. & Rantalainen, M. (2021). Predicting molecular phenotypes from histopathology images: a transcriptome-wide expression–morphology analysis in breast cancer. Cancer research, 81(19), 5115-5126.
Close collaborators:
Prof. Minna Kaikkonen-Määttä
University of Eastern Finland
Adj. Prof. Tapio Lönnberg
Turku Bioscience Center
Prof. Merja Heinäniemi
University of Eastern Finland
3D reconstruction, analysis and visualization of multimodal data
Understanding the mechanisms of diseases requires resolving the spatial context of measurements. While the spatial context is increasingly included in studies of 2D samples, the tissues originate from organs that are 3D. Ignoring the spatial surroundings in 3D may lead to unideal view of the local environment of cells and tissue, limiting the capability to decipher and understand the interplay of cells in their natural 3D environment. Here, we set out to develop methods and tools to reconstruct and analyze 3D spatial structure from multimodal measurements including tissue with multiple stainings, as well as other spatially resolved measurements and genomics. Further, we will pay special attention to visualization of multimodal data and quantitative feature representations of the tissue. Our prior work in this area includes benchmarking of multistained tissue registration, 3D reconstruction from serial sections, analysis of 3D histology, and virtual reality (VR) system for visualization. Here, we seek to develop there computational tools further and to integrate genomics and other spatial measurements using the tools to gain novel insights into the spatial environment of aorta in 3D.
Our research group has extensive experience in biomedical image analysis, especially in digital pathology. We build computational systems using modern machine learning and artificial intelligence and utilize high-performance computing. We are a multinational team of enthusiastic and skilled computer scientists with diverse cultural background. Our group operates in close collaboration with biologists, pathologists, medical doctors and bioinformaticians, and our collaboration network consists of highly ranked researchers and laboratories from Finland and internationally. We are part of the immunology related InFlames flagship.
We are looking for highly motivated PhD with degree from computer science or related field, and experience from computational biology and image analysis. Successful candidate has skills in Python programming and ability to handle large datasets as well as run large-scale computing. Previous experience from VR or other visualization tools is a benefit. The role of the post-doctoral researcher is to lead computational method development and to operate in close collaboration in a highly multidisciplinary and international team with outstanding collaborators who are leading experts in cardiovascular genomics, multimodal imaging, and cell and cancer biology.
Key words:
3D, VR, AI, histology, genomics, spatial analysis, multimodal data, cardiovascular disease, atherosclerosis
Selected publications:
- Ruusuvuori, P., Valkonen, M., Kartasalo, K., Valkonen, M., Visakorpi, T., Nykter, M., & Latonen, L. (2022). Spatial analysis of histology in 3D: quantification and visualization of organ and tumor level tissue environment. Heliyon, 8(1).
- Liimatainen, K., Latonen, L., Valkonen, M., Kartasalo, K., & Ruusuvuori, P. (2021). Virtual reality for 3D histology: multi-scale visualization of organs with interactive feature exploration. BMC cancer, 21, 1-14.
- Latonen, L., Koivukoski, S., Khan, U., & Ruusuvuori, P. (2024). Virtual staining for histology by deep learning. Trends in Biotechnology.
Close collaborators:
Adj. Prof. Leena Latonen
University of Eastern Finland
Prof. Anne Roivainen
University of Turku
Prof. Minna Kaikkonen-Määttä
University of Eastern Finland
Ultrasensitive immunoassays for neurological biomarkers
Currently the practical use of protein biomarkers for neurological diseases is significantly limited due to their presence in blood at hardly detectable or totally undetectable levels using conventional immunoassay technologies, which are routinely used for other biomarkers. The neurological biomarkers can be studied using cerebral spinal fluid, but measurement of proteins leaking through the blood-brain barrier would enable significantly less invasive blood sampling. Thus, the availability of ultrasensitive immunoassays would facilitate convenient testing and support development of neurological biomarkers to earlier diagnostics of neurological disorders.
We have developed in collaboration with European wide research network a new superior luminescent reporter technology, photon upconversion luminescence, and demonstrated its capability to ultrasensitive detection at conventional immunoassay platform matching the sensitivity of even the most complex digital immunoassays based on special instrumentation. Photon upconversion is based on trivalent lanthanide doped nanomaterials capable of stacking the energy of two absorbed low-energy near-infrared photons to emit a single high-energy photon at visible wavelengths. The resulting anti-Stokes photoluminescence, which is measurable using inexpensive epifluorescence setup, enables total elimination of background autofluorescence and results in exceptionally low limit of detection in immunoassays for protein biomarkers.
The limit-of-detection in photon upconversion luminescence-based assays is still fundamentally restricted by the specific binding strength of the employed antibodies and the non-specific binding of the reporter-antibody conjugates. To enable further improvement of the assay sensitivity we are currently studying active techniques and countermeasures to reduce and eliminate the signal originating from the non-specific interactions of the reporter-antibody conjugates. These techniques could enable a breakthrough in the development of ultrasensitive immunoassays using photon upconversion luminescence.
Key words:
Digital immunoassay, In vitro diagnostics, Diagnostic discovery, Neurological disorders, Neurological biomarkers, Blood test limit-of-detection, Blood-brain barrier leakage, photon upconversion luminescence
Selected publications:
- What digital immunoassays can learn from ambient analyte theory: a perspective. Hans H Gorris, Tero Soukka. Anal Chem 2022 94:6073-6083. doi: 10.1021/acs.analchem.1c05591.
- Supersensitive photon upconversion based immunoassay for detection of cardiac troponin I in human plasma. Raiko, K., Lyytikäinen A, Ekman M, Nokelainen A, Lahtinen S, Soukka T. Clin Chim Acta. 2021; 523:380-385. doi: 10.1016/j.cca.2021.10.023.
- Effect of particle size and surface chemistry of photon-upconversion nanoparticles on analog and digital Immunoassays for cardiac troponin. Brandmeier JC, Raiko K, Farka Z, Peltomaa R, Mickert MJ, Hlaváček A, Skládal P, Soukka T, Gorris HH. Adv Healthc Mater. 2021; 10:e2100506. doi: 10.1002/adhm.202100506.
- Improving the sensitivity of immunoassays by reducing non-specific binding of poly(acrylic acid) coated upconverting nanoparticles by adding free poly(acrylic acid). Lahtinen S, Lyytikäinen A, Sirkka N, Päkkilä H, Soukka T. Mikrochim Acta. 2018; 185:220. doi: 10.1007/s00604-018-2756-z.
Instrumentation and Image Processing (IntoImaging) Research Group
We are looking for an active post-doctoral researcher to join our Instrumentation and Image Processing team (IntoImaging research group: https://sites.utu.fi/intoimaging/en/). Our team is a technically oriented focusing on methodological research in PET imaging, working with a close collaboration with the other clinical and research teams, including cardiometabolic and brain health in Turku PET Centre. You will be tightly working with a multi-disciplinary team consisting of people with backgrounds in medicine, engineering, physics and modelling.
Our main research focus is PET and other medical imaging techniques, with the two main clinical applications focusing tightly on the fields in cardiovascular and brain imaging. Especially, we investigate PET image reconstruction methods, including attenuation and motion correction. We also develop image processing tools for medical purposes, including machine learning methods. Some of our research topics include but are not limited to: Medical tomography image analysis using AI, Motion correction of PET images, MRI based attenuation correction of PET images, Image quantification accuracy, New kinetic modelling approaches for total body PET and New tools for medical image analysis and diagnostics.
In our group, you would be participating to grounds-up methods development: by developing methods to improve the quantitative accuracy of PET, development of new tools for modelling and analysis of PET images, investigation of the accuracy and effect of different methodological factors affecting PET image quantification and determining whether application of AI would bring a significant benefit in any of these sub-topics. Our goal is to develop methods to improve the quantitative accuracy, applicability and effectiveness of PET imaging in cardiometabolic and brain studies as well as enabling efficient analysis of such data.
We are looking for candidates with the relevant background, experience and PhD degree in medicine, medical physics, biomedical engineering, or related backgrounds e.g. computational science to apply. Independent of the background of the applicant, understanding and proven track record (e.g. by publications) from PET imaging and method development is required.
We value good programming skills in Python and MATLAB, require ability to work in a multi-disciplinary team and to have good and active communication and teamwork skills. Work experience from a clinical environment, or a clinical background is considered a plus. We are dedicated to host researchers who strive for academic excellence and are devoted in helping them to excel in their research careers by providing them the best possible grounds for their research as well as direction and mentoring. The suitable applicants are engouraged to propose research ideas and preliminary research plans according to the research topics mentioned above.
Key words:
PET imaging, image quantification, motion correction, modelling, machine learning
Selected publications:
- Teuho J, Schultz J, Klén R, Juarez-Orozco LE, Knuuti J, Saraste A, Ono N, Kanaya S. Explainable deep learning-based ischemia detection using hybrid O-15 H2O perfusion PET/CT imaging and clinical data. 2024, Journal of Nuclear Cardiology.
- Jafaritadi M, Teuho J, Lehtonen E, Klén R, Saraste A, CS Levin. Deep Generative Denoising Networks Enhance Quality and Accuracy of Gated Cardiac PET Data. 2024, Annals of Nuclear Medicine.
- Siekkinen R, Partanen H, Kukola L, Tolvanen T, Fenwick A, Smith NAS, Teräs M, Saraste A, Teuho J. Preliminary Protocol for Measuring the Reproducibility and Accuracy of Flow values on Digital PET/CT Systems in [15O]H2O Myocardial Perfusion Imaging Using a Flow Phantom. 2024, EJNMMI Physics.
- Knuuti J, Tuisku J, Kärpijoki H, Iida H, Maaniitty T, Latva-Rasku A, Oikonen V, Nesterov SV, Teuho J, Jaakkola MK, Klén R, Louhi H, Saunavaara V, Nuutila P, Saraste A, Rinne J, Nummenmaa L. Quantitative Perfusion Imaging with Total-Body PET. 2023, Journal of Nuclear Medicine.
- Siekkinen R, Chunlei H, Maaniitty T, Teräs M, Knuuti J, Saraste A*, Teuho J* A retrospective evaluation of Bayesian-penalized likelihood reconstruction for [15O]H2O myocardial perfusion imaging, 2023, Journal of Nuclear Cardiology. *equal contribution.
- Rainio O, Han C, Teuho J, Nesterov SV, Oikonen V, Piirola S, Laitinen T, Tättäläinen M, Knuuti J, Klén R. Carimas: An Extensive Medical Imaging Data Processing Tool for Research. 2023, J. Digit. Imaging.
- Teuho J, Schultz J, Klén R, Saraste A, Ono N, Kanaya S. Classification of ischemia from myocardial polar maps in 15O-H2O cardiac perfusion imaging using a convolutional neural network. 2022, Scientific Reports
- Schultz J, Siekkinen R, Jafari Tadi M, Teräs M, Klén R, Lehtonen E, Saraste A, Teuho J. Effect of respiratory motion correction and CT-based attenuation correction on dual-gated cardiac PET im-age quality and quantification, 2022. J Nucl Cardiol.
- Siekkinen R, Kirjavainen AK, Koskensalo K, Smith NAS, Fenwick A, Saunavaara V, Tolvanen T, Iida H, Saraste A, Teräs M, Teuho J, Assessment of a Digital and an Analog PET/CT System for Accurate Myocardial Perfusion Imaging with a Flow Phantom, 2021, Journal of Nuclear Cardiology.
- Gambin JR, Tadi MJ, Teuho J, Klen R, Knuuti J, Koskinen J, et al. Learning to Denoise Gated Cardiac PET Images Using Convolutional Neural Networks, 2021, IEEE Access.
Jarmo Teuho
Docent (Adj. Prof) in Medical Imaging Physics and Technology
PhD in Medical Physics and Engineering
Academy Research Fellow, Turku PET Centre
In your contact email please indicate clearly in the headline your interest in the SYSLIFE position.
Investigators
Docent Jarmo Teuho, PhD
Associate Professor Riku Klén, PhD
In vitro diagnostic tests for cardiac biomarkers
Laboratory tests are being used around the world for diagnosing cardiac diseases and estimating the risk for adverse cardiac events. However, these tests are not optimal and tests with better clinical specificity and sensitivity are needed to improve patient care. At the Cardiac Biomarker Laboratory, we aim to develop laboratory tests for novel cardiac biomarkers that will have significant impact on how cardiac diseases are diagnosed and treated.
The Cardiac Biomarker Laboratory of the Biotechnology Unit at the Department of Life Technologies is focused on developing highly sensitive immunoassays for novel cardiac biomarkers and investigating the clinical use of these biomarkers for diagnosis and risk prediction of cardiac diseases. The group, led by Assistant Professor Saara Wittfooth, applies the innovative label technologies developed at the Biotechnology Unit to develop unique assays that are not available in any other laboratory (examples of such assays are the assays for long cardiac troponin T, free PAPP-A and cardiac troponin autoantibodies). The work includes developing immunoassays in various platforms by designing antibody combinations and optimizing assay conditions and procedures. The work involves close collaboration with cardiologists around the world.
Lately the research of the Laboratory has been focused on different forms of cardiac troponins. Tests for cardiac troponins (cardiac troponin I and cardiac troponin T, cTnT) are widely used for diagnosing myocardial infarction around the world. However, the high sensitivity troponin tests that are currently used in the hospitals produce elevated results also in many other situations than myocardial infarction (such as impaired kidney function, atrial fibrillation, severe infections, strenuous physical exercise). Therefore, there is a high demand for a more specific diagnostic test for myocardial infarction. We have developed in the Cardiac Biomarker Laboratory a novel simple immunoassay-based test that detects only the long forms of cTnT, while the tests being used at hospitals detect the long and short forms of cTnT. Our first studies have shown very promising results, as the long cTnT test was able to differentiate between myocardial infarction patients and end-stage renal disease patients much better than the troponin test currently in use at hospitals.
Another research topic gaining more interest after a while in the Cardiac Biomarker Laboratory is the cardiac troponin autoantibodies (cTnAAb). cTnAAb, which have been found in the blood of myocardial infarction patients but also in healthy individuals, can cause negative interference in immunoassays by masking the epitopes of the assay antibodies. An assay to detect cTnAAb in patient samples was previously developed in the Laboratory. Lately there has been increasing interest in the scientific community towards macrotroponins, circulating complexes of troponins and cTnAAb, which may cause positive interference due to reduced clearance. We are now interested in examining macrotroponins with the available cTnAAb assay as well as with other methods in various patient groups.
Key words:
In vitro diagnostics, Myocardial infarction, Cardiac biomarkers, Cardiac troponins, Troponin autoantibodies
The candidate should have:
- PhD in a suitable field (biotechnology, biochemistry, laboratory medicine etc.)
- Extensive experience in performing and developing immunoassays
- Ability to communicate fluently in English
- Excellent scientific writing skills
Selected publications:
- Salonen SM, Tuominen TJK, Raiko KIS, Vasankari T, Aalto R, Hellman TA, Lahtinen SE, Soukka T, Airaksinen KEJ, Wittfooth ST. Highly Sensitive Immunoassay for Long Forms of Cardiac Troponin T Using Upconversion Luminescence. Clin Chem. 2024 Aug 1;70(8):1037-1045. doi: 10.1093/clinchem/hvae075
- Airaksinen KEJ, Aalto R, Hellman T, Vasankari T, Lahtinen A, Wittfooth S. Novel Troponin Fragmentation Assay to Discriminate Between Troponin Elevations in Acute Myocardial Infarction and End-Stage Renal Disease. Circulation. 2022 Nov;146(18):1408-1410. doi: 10.1161/CIRCULATIONAHA.122.060845
- Savukoski T, Ilva T, Lund J, Porela P, Ristiniemi N, Wittfooth S, Pettersson K. Autoantibody prevalence with an improved immunoassay for detecting cardiac troponin-specific autoantibodies. Clin Chem Lab Med. 2014 Feb;52(2):273-9. doi: 10.1515/cclm-2013-0310.
- Savukoski T, Twarda A, Hellberg S, Ristiniemi N, Wittfooth S, Sinisalo J, Pettersson K. Epitope specificity and IgG subclass distribution of autoantibodies to cardiac troponin. Clin Chem. 2013 Mar;59(3):512-8. doi: 10.1373/clinchem.2012.194860.
