Novel research on atrial fibrillation - CAREFIB
We can host a skillful and motivated scientist with a PhD degree in cell biology, biochemistry or medicine. Our group enables studies related to cardiovascular disease such as atherosclerosis, coronary artery disease, aortic valve calcification, aortic regurgitation, mitral valve disease and atrial fibrillation. The CAREFIB project aims to advance the understanding of the cellular and inflammatory origin of cardiovascular disease (mostly focused on but not limited to atrial fibrillation) and thereby allow development of specific treatment for this disorder. The project focuses especially on deep cellular phenotyping of atrial fibrillation using human samples. It utilizes the vast human heart sample collection (n=1,001 patients) with sn-RNAseq data aligned with tissue and serum/plasma proteomics data as well as the possibility to use zebrafish core. The research is executed between the Heart Center at the Turku University Hospital and Medicity Research Laboratory at the University of Turku.
In our CAREFIB Team, we appreciate ability to work as part of a team and strong motivation for science, as well as excellent writing and oral communication skills in English.
Key words:
Inflammation, Cardiovascular diseases, Atrial fibrillation, Atherosclerosis, Coronary artery disease, Aortic valve calcification, Aortic regurgitation, Mitral valve disease, RNA-seq analysis, Proteomic analysis, Transcriptomic analysis, Image analysis, Target validation
Advanced imaging in coronary artery disease
The cardiovascular research team at Turku PET Centre and Heart Center has vast experience on non-invasive, multi-modality imaging of coronary artery disease. The current projects investigate the use of coronary computed tomography, positron emission tomography imaging and echocardiography for the detection of non-obstructive and obstructive disease as well as the outcome. The research utilise national and international patient cohorts and advanced image analysis including machine learning.
Non-invasive imaging of coronary artery disease (CAD) has been rapidly advancing. Positron emission tomography can measure myocardial perfusion quantitatively. X-ray coronary computed tomography angiography (CCTA) enables detailed visualisation of coronary plaques and stenoses. The combination of both anatomy and function has been shown to be ideal for assessing prognosis and guide therapy decisions.
At the same time the analysis of image data has become more demanding and time-consuming. Recently, machine learning techniques appears to enhance the analysis process. Our project aims to generate an adaptive and self-sustaining workflow for the collection, storage, integration, analysis and continuous update of available patient clinical, imaging and genetic cardiovascular data in Turku, Finland, Amsterdam, Leiden and Utrecht, the Netherlands. The second objective is to incorporate, train and validate a range of statistical machine learning algorithms on the aforementioned data in order to offer support estimations in the diagnosis and outcome prediction of patients with known or suspected CAD.
The current work is based on the access to large cohorts of patients with CCTA, PET perfusion imaging, clinical data and nearly 10 years of follow-up. The cohorts include several thousands of patients from Turku and sites in the Netherlands already partly pooled together. The analysis includes all recently introduced advanced CCTA methods (e.g. plaque composition, shear stress, Fat attenuation index). Regional quantitation of myocardial perfusion has been also already performed. In addition to our home-developed machine learning processed we have collaborated with industry for AI-based analyses of CCTA images.
We believe that the outcomes of the project will increase the accuracy of diagnostics and predictive power of available data allowing highly-individualized risk based therapy guidance, and ultimately, to improve cardiovascular disease-related outcomes.
Successful candidates should have a background and PhD degree in medicine, biomedicine, or related fields, and skills for working in multidisciplinary team as well as experience on cardiac imaging and advanced data and statistical analyses.
Key Words:
Cardiac imaging, coronary artery disease diagnostics, PET perfusion imaging, CCTA, machine learning, AI-based image analysis
Figure. Hybrid heart image using CCTA and PET perfusion imaging. The 3D rendered CCTA images were merged with quantitative perfusion images with O-15-water PET.
Selected publications:
- Lehtonen E, Kujala I, Tamminen J, Maaniitty T, Saraste A, Teuho J, Knuuti J, Klén R. Incremental prognostic value of downstream PET perfusion imaging after coronary CT angiography. Eur Heart J Cardiovasc Imaging. 2023 Sep 29:jead246. doi: 10.1093/ehjci/jead246. Epub ahead of print. PMID: 37774503.
- Juarez-Orozco LE, Niemi M, Yeung MW, Benjamins JW, Maaniitty T, Teuho J, Saraste A, Knuuti J, van der Harst P, Klén R. Hybridizing machine learning in survival analysis of cardiac PET/CT imaging. J Nucl Cardiol. 2023 Sep 1. doi: 10.1007/s12350-023-03359-4. Epub ahead of print. PMID: 37656345
- van Diemen PA, de Winter RW, Schumacher SP, Everaars H, Bom MJ, Jukema RA, Somsen YB, Raijmakers PG, Kooistra RA, Timmer J, Maaniitty T, Robbers LF, von Bartheld MB, Demirkiran A, van Rossum AC, Reiber JH, Knuuti J, Underwood RS, Nagel E, Knaapen P, Driessen RS, Danad I. The Diagnostic Performance of QFR and Perfusion Imaging in Patients with Prior Coronary Artery Disease. Eur Heart J Cardiovasc Imaging. 2023 Aug 14:jead197. doi: 10.1093/ehjci/jead197. Epub ahead of print. PMID: 37578007.
- Mäenpää M, Kujala I, Harjulahti E, Stenström I, Nammas W, Knuuti J, Saraste A, Maaniitty T. The impact of diabetes on the relationship of coronary artery disease and outcome: a study using multimodality imaging. Cardiovasc Diabetol. 2023 May 31;22(1):129. doi: 10.1186/s12933-023-01850-3. PMID: 37254111; PMCID: PMC10230727.
- Kujala I, Nammas W, Maaniitty T, Stenström I, Klén R, Bax JJ, Knuuti J, Saraste A. Prognostic value of combined coronary CT angiography and myocardial perfusion imaging in women and men. Eur Heart J Cardiovasc Imaging. 2023 Aug 23;24(9):1201-1209. doi: 10.1093/ehjci/jead072. PMID: 37086269; PMCID: PMC10445260.
- Kuneman JH, van den Hoogen IJ, Schultz J, Maaniitty T, van Rosendael AR, Kamperidis V, de Graaf MA, Broersen A, Jukema JW, Bax JJ, Saraste A, Knuuti J. Plaque volume, composition, and fraction versus ischemia and outcomes in patients with coronary artery disease. J Cardiovasc Comput Tomogr. 2023 May- Jun;17(3):177-184. doi: 10.1016/j.jcct.2023.02.004. Epub 2023 Mar 13. PMID: 36922308.
- Jukema R, Maaniitty T, van Diemen P, Berkhof H, Raijmakers PG, Sprengers R, Planken RN, Knaapen P, Saraste A, Danad I, Knuuti J. Warranty period of coronary computed tomography angiography and [15O]H2O positron emission tomography in symptomatic patients. Eur Heart J Cardiovasc Imaging. 2023 Feb 17;24(3):304-311. doi: 10.1093/ehjci/jeac258. PMID: 36585755.
- Schultz J, van den Hoogen IJ, Kuneman JH, de Graaf MA, Kamperidis V, Broersen A, Jukema JW, Sakellarios A, Nikopoulos S, Tsarapatsani K, Naka K, Michalis L, Fotiadis DI, Maaniitty T, Saraste A, Bax JJ, Knuuti J. Coronary computed tomography angiography-based endothelial wall shear stress in normal coronary arteries. Int J Cardiovasc Imaging. 2023 Feb;39(2):441-450. doi: 10.1007/s10554-022-02739-0. Epub 2022 Oct 18. PMID: 36255544; PMCID: PMC9870961.
- Wang X, van den Hoogen IJ, Butcher SC, Kuneman JH, de Graaf MA, Kamperidis V, Boukes M, Maaniitty T, Schultz J, van Rosendael AR, Saraste A, Knuuti J, Bax JJ. Importance of plaque volume and composition for the prediction of myocardial ischaemia using sequential coronary computed tomography angiography/positron emission tomography imaging. Eur Heart J Cardiovasc Imaging. 2023 May 31;24(6):776-784. doi: 10.1093/ehjci/jeac130. PMID: 36047438; PMCID: PMC10229289.
- Van den Hoogen IJ, Schultz J, Kuneman JH, de Graaf MA, Kamperidis V, Broersen A, Jukema JW, Sakellarios A, Nikopoulos S, Kyriakidis S, Naka KK, Michalis L, Fotiadis DI, Maaniitty T, Saraste A, Bax JJ, Knuuti J. Detailed behaviour of endothelial wall shear stress across coronary lesions from non-invasive imaging with coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2022 Nov 17;23(12):1708-1716. doi: 10.1093/ehjci/jeac095. PMID: 35616068; PMCID: PMC10017098.
- Maaniitty T, Stenström I, Saraste A, Knuuti J. Extensive and balanced reduction of myocardial blood flow in patients with suspected obstructive coronary artery disease: 15O-water PET study. Int J Cardiol. 2021 Sep 1;338:1-7. doi: 10.1016/j.ijcard.2021.06.012. Epub 2021 Jun 16. PMID: 34144073.
- Harjulahti E, Maaniitty T, Nammas W, Stenström I, Biancari F, Bax JJ, Knuuti J, Saraste A. Global and segmental absolute stress myocardial blood flow in prediction of cardiac events: [15O] water positron emission tomography study. Eur J Nucl Med Mol Imaging. 2021 May;48(5):1434-1444. doi: 10.1007/s00259-020-05093-2. Epub 2020 Nov 11. PMID: 33174090; PMCID: PMC8113164.
- Stenström I, Maaniitty T, Uusitalo V, Ukkonen H, Kajander S, Mäki M, Nammas W, Bax JJ, Knuuti J, Saraste A. Absolute Stress Myocardial Blood Flow After Coronary CT Angiography Guides Referral to Invasive Angiography. JACC Cardiovasc Imaging. 2019 Nov;12(11 Pt 1):2266-2267. doi: 10.1016/j.jcmg.2019.08.002. Epub 2019 Sep 18. PMID: 31542538.
- Maaniitty T, Jaakkola S, Saraste A, Knuuti J. Hybrid coronary computed tomography angiography and positron emission tomography myocardial perfusion imaging in evaluation of recurrent symptoms after coronary artery bypass grafting. Eur Heart J Cardiovasc Imaging. 2019 Nov 1;20(11):1298-1304. doi: 10.1093/ehjci/jey160. PMID: 30388202.
- Stenström I, Maaniitty T, Uusitalo V, Pietilä M, Ukkonen H, Kajander S, Mäki M, Bax JJ, Knuuti J, Saraste A. Frequency and angiographic characteristics of coronary microvascular dysfunction in stable angina: a hybrid imaging study. Eur Heart J Cardiovasc Imaging. 2017 Nov 1;18(11):1206-1213. doi: 10.1093/ehjci/jex193. PMID: 28950300.
- Dimitriu-Leen AC, van Rosendael AR, Smit JM, van Elst T, van Geloven N, Maaniitty T, Jukema JW, Delgado V, Scholte AJHA, Saraste A, Knuuti J, Bax JJ. Long-Term Prognosis of Patients With Intramural Course of Coronary Arteries Assessed With CT Angiography. JACC Cardiovasc Imaging. 2017 Dec;10(12):1451-1458. doi: 10.1016/j.jcmg.2017.02.013. Epub 2017 May 17. PMID: 28528150.
- Maaniitty T, Stenström I, Bax JJ, Uusitalo V, Ukkonen H, Kajander S, Mäki M, Saraste A, Knuuti J. Prognostic Value of Coronary CT Angiography With Selective PET Perfusion Imaging in Coronary Artery Disease. JACC Cardiovasc Imaging. 2017 Nov;10(11):1361-1370. doi: 10.1016/j.jcmg.2016.10.025. Epub 2017 May 17. PMID: 28528146.
Juhani Knuuti
Professor, Director, Turku PET Centre
Investigators
Principal investigators:
Professor Juhani Knuuti, MD, PhD
Professor Antti Saraste, MD, PhD
Senior investigators:
Teemu Maaniitty
Sergey Nesterov
Jarmo Teuho
Maria Jaakkola
Riku Klén
PhD students:
Sarah Bär
Iida Kujala
Matias Mäenpää
Esa Harjulahti
Christian Paunonen
Sauli Uotila
Research coordinator:
Heli Louhi
Quantitative Perfusion Imaging with Total-Body PET
The cardiovascular research team at Turku PET Centre and Heart Center is developing and investigating non-invasive quantitative total-body perfusion imaging using a very short-living PET tracer, oxygen-15 labelled water. The project includes both technological development as well as clinical applications of the method.
Recently, long axial field of view PET systems have become the current state of the art. Total-body PET scanners enable unique possibilities for scientific research and clinical diagnostics, but this new technology also raises numerous challenges. A key advantage of the total body imaging is that having all the organs in the field of view allows studying biological interaction of all organs simultaneously. One of the new promising imaging targets is the total body quantitative perfusion imaging. Currently, oxygen-15 labelled water provides feasible option for absolute quantitation of tissue perfusion at total body level. The basic method development utilising also machine learning is well advancing and clinical study in patients with suspected coronary artery disease well advancing. The current findings encourage to expand the method into other relevant patient populations.
The research is done in collaboration with the Heart Center of Turku University Hospital and Emotion lab at Turku PET Centre. The goal is to improve our understanding on interaction between organs in various cardiovascular diseases and conditions. The ultimate goal is to develop total-body perfusion imaging as a tool for clinical diagnostics.
Successful candidates should have a background and PhD degree in medicine, computational science, physics, or related fields, and skills for working with complex environment and multidisciplinary team.
Key words:
total body PET, perfusion imaging, oxygen-15 tracer, quantitative imaging, cardiovascular diseases
Selected publications:
- Knuuti J, Tuisku J, Kärpijoki H, Iida H, Maaniitty T, Latva-Rasku A, Oikonen V, Nesterov SV, Teuho J, Jaakkola MK, Klén R, Louhi H, Saunavaara V, Nuutila P, Saraste A, Rinne J, Nummenmaa L. Quantitative Perfusion Imaging with Total-Body PET. J Nucl Med. 2023 Nov;64(Suppl 2):11S-19S. doi: 10.2967/jnumed.122.264870. PMID: 37918848.
- Kajander SA, Joutsiniemi E, Saraste M, et al. Clinical Value of Absolute Quantification of Myocardial Perfusion With 15O-Water in Coronary Artery Disease. Circ Cardiovasc Imaging. 2011;4:678-684.
- Maaniitty T, Stenström I, Saraste A, Knuuti J. Extensive and balanced reduction of myocardial blood flow in patients with suspected obstructive coronary artery disease: 15O-water PET study. Int J Cardiol. 2021;338:1-7.
- Maaniitty T, Knuuti J, Saraste A. 15O-Water PET MPI: Current Status and Future Perspectives. Semin Nucl Med. 2020;50:238-247.
- Iida H, Kanno I, Takahashi A, et al. Measurement of absolute myocardial blood flow with H215O and dynamic positron-emission tomography. Strategy for quantification in relation to the partial-volume effect. Circulation. 1988;78:104-115.
- Iida H, Tamura Y, Kitamura K, Bloomfield PM, Eberl S, Ono Y. Histochemical Correlates of 15 O-Water-Perfusable Tissue Fraction in Experimental Canine Studies of Old Myocardial Infarction. J Nucl Med. 2000;41:1737-1745.
- Iida H, Rhodes CG, De Silva R, et al. Myocardial tissue fraction--correction for partial volume effects and measure of tissue viability. J Nucl Med. 1991;32:2169-2175.

Figure. One of the first cases of total body parametric images with 15O-water shown by Carimas software. The images are processed to three 3D parametric components: tissue perfusion, perfusable tissue fraction and arterial blood volume images. Delay and dispersion corrections were not applied, and the same model was used in all organs. Therefore, these parametric images are not yet ideal, e.g., K1 is influenced by the arterial vessels largely attributed to the delay and dispersion.
Juhani Knuuti
Professor, Director, Turku PET Centre
Investigators
Principal investigators:
Professor Juhani Knuuti, MD, PhD
Professor Antti Saraste, MD, PhD
Senior investigators:
Hidehiro Iida
Jouni Tuisku
Teemu Maaniitty
Sergey Nesterov
Jarmo Teuho
Maria Jaakkola
Riku Klén
Virva Saunavaara
Juha Rinne
Lauri Nummenmaa
PhD students:
Henri Kärpijoki
Sarah Bär
Iida Kujala
Matias Mäenpää
Esa Harjulahti
Research coordinator:
Heli Louhi
Molecular imaging in heart diseases
The cardiovascular research team at Turku PET Centre and Heart Center is one of the leading research groups on molecular imaging in heart diseases. The focus has been on coronary artery disease and heart failure. The current projects investigate the non-invasive imaging of cardiac remodelling and inflammation in various clinical conditions.
Positron emission tomography can detect and quantify pathophysiological processes underlying heart failure, complementing evaluation of cardiac structure and function with other imaging modalities. High sensitivity of nuclear imaging to detect targeted tracers has enabled assessment of various cellular mechanisms of heart failure. Nuclear imaging of active inflammation and amyloid deposition are incorporated into clinical management algorithms of cardiac sarcoidosis and amyloidosis. Emerging tracers specific for inflammation and early stages of myocardial fibrosis are in earlier stages of development, but have demonstrated potential value in early detection of myocardial damage and prediction of cardiac dysfunction. Early detection of disease activity is a key for transition from treatment of heart failure to personalized therapy targeted to prevent heart failure.
The research done in collaboration with the Turku PET Centre and Heart Center of Turku University Hospital is based on unique access to numerous PET tracers fur animal and human use as well as and advanced multimodality imaging (PET, CT, MRI, ultrasound). The ongoing projects of the cardiovascular team investigate non-invasive imaging of cardiac remodelling and inflammation in various clinical conditions.
Successful candidates should have a background and PhD degree in medicine, biomedicine, or related fields, and skills for working in multidisciplinary team as well as experience on cardiovascular molecular imaging, either in animals or humans.
Key words:
Molecular imaging, PET, CT, MRI, ultrasound, inflammation, cardiac remodelling
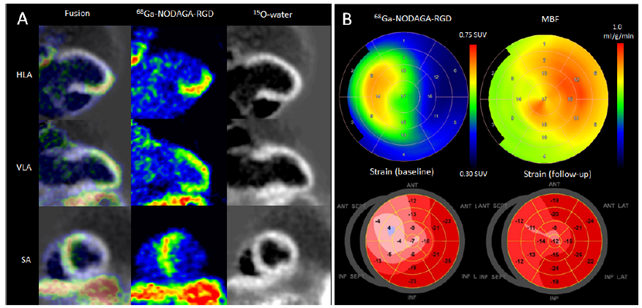
Figure. Uptake of [68Ga]Ga-NODAGA-RGD 7 days after acute occlusion of the proximal left anterior descending coronary artery. Panel A shows myocardial contours in [15O]O-water images, [68Ga]Ga-NODAGA-RGD uptake images, and corresponding fusion images. Panel B shows polar maps of [68Ga]Ga-NODAGA-RGD uptake, resting myocardial blood flow (MBF), and longitudinal strain at the time of PET and 6 months later. Note the reduced longitudinal strain in the anteroseptal region at baseline and partial functional recovery at 6 months. HLA=horizontal long axis, SA=short axis, SUV=standardized uptake value, VLA=vertical long axis. (Nammas et al. J Nucl Med 2023)
Selected publications:
- Saraste A, Knuuti J, Bengel F. Phenotyping heart failure by nuclear imaging of myocardial perfusion, metabolism, and molecular targets. Eur Heart J Cardiovasc Imaging. 2023 Sep 26;24(10):1318-1328. doi: 10.1093/ehjci/jead128. PMID: 37294318; PMCID: PMC10531130.
- Jahandideh A, Uotila S, Ståhle M, Virta J, Li XG, Kytö V, et al. Folate Receptor β-Targeted PET Imaging of Macrophages in Autoimmune Myocarditis. J Nucl Med. 2020;61:1643-1649.
- Grönman M, Tarkia M, Kiviniemi T, Halonen P, Kuivanen A, Savunen T, et al. Imaging of αvβ3 integrin expression in experimental myocardial ischemia with [68Ga]NODAGA-RGD positron emission tomography. J Transl Med. 2017;15:144.
- Nammas W, Paunonen C, Teuho J, Siekkinen R, Luoto P, Käkelä M, Hietanen A, Viljanen T, Dietz M, Prior JO, Li XG, Roivainen A, Knuuti J, Saraste A. Imaging of Myocardial αvβ3 Integrin Expression for Evaluation of Myocardial Injury After Acute Myocardial Infarction. J Nucl Med. 2023 Nov 16:jnumed.123.266148. doi: 10.2967/jnumed.123.266148. Epub ahead of print. PMID: 37973184.
- Jahandideh A, Virta J, Li XG, Liljenbäck H, Moisio O, Ponkamo J, Rajala N, Alix M, Lehtonen J, Mäyränpää MI, Salminen TA, Knuuti J, Jalkanen S, Saraste A, Roivainen A. Vascular adhesion protein-1-targeted PET imaging in autoimmune myocarditis. J Nucl Cardiol. 2023 Sep 27. doi: 10.1007/s12350-023-03371-8. Epub ahead of print. PMID: 37758963.
- Palani S, Miner MWG, Virta J, Liljenbäck H, Eskola O, Örd T, Ravindran A, Kaikkonen MU, Knuuti J, Li XG, Saraste A, Roivainen A. Exploiting Glutamine Consumption in Atherosclerotic Lesions by Positron Emission Tomography Tracer (2S4R)-4-18F-Fluoroglutamine. Front Immunol. 2022 Jan 25;13:821423. doi: 10.3389/fimmu.2022.821423. Erratum in: Front Immunol. 2022 Apr 12;13:902544. PMID: 35145523; PMCID: PMC8822173.
- Ståhle M, Hellberg S, Virta J, Liljenbäck H, Metsälä O, Li XG, Jauhiainen M, Saukko P, Ylä-Herttuala S, Nuutila P, Knuuti J, Saraste A, Roivainen A. Evaluation of glucagon-like peptide-1 receptor expression in nondiabetic and diabetic atherosclerotic mice using PET tracer 68Ga-NODAGA-exendin-4. Am J Physiol Endocrinol Metab. 2021 May 1;320(5):E989-E998. doi: 10.1152/ajpendo.00465.2020. Epub 2021 Apr 12. PMID: 33843281.
- Virta J, Hellberg S, Liljenbäck H, Ståhle M, Silvola JMU, Huusko J, Söderström M, Knuuti J, Nuutila P, Ylä-Herttuala S, Gomez MF, Roivainen A, Saraste A. Effects of dipeptidyl peptidase 4 inhibition on inflammation in atherosclerosis: A 18F-fluorodeoxyglucose study of a mouse model of atherosclerosis and type 2 diabetes. Atherosclerosis. 2020 Jul;305:64-72. doi: 10.1016/j.atherosclerosis.2020.03.029. Epub 2020 Apr 10. PMID: 32386751.
- Ståhle M, Silvola JMU, Hellberg S, de Vries M, Quax PHA, Kroon J, Rinne P, de Jong A, Liljenbäck H, Savisto N, Wickman A, Stroes ESG, Ylä-Herttuala S, Saukko P, Abrahamsson T, Pettersson K, Knuuti J, Roivainen A, Saraste A. Therapeutic Antibody Against Phosphorylcholine Preserves Coronary Function and Attenuates Vascular 18F-FDG Uptake in Atherosclerotic Mice. JACC Basic Transl Sci. 2020 Mar 25;5(4):360-373. doi: 10.1016/j.jacbts.2020.01.008. PMID: 32368695; PMCID: PMC7188869.
- Stolen KQ, Kemppainen J, Kalliokoski KK, Hallsten K, Luotolahti M, Karanko H et al., Myocardial perfusion reserve and oxidative metabolism contribute to exercise capacity in patients with dilated cardiomyopathy. J Card Fail. 2004;10:132–140.
